Alkanes
An alkane is a hydrocarbon that only contains single carbon-carbon bonds. Alkanes constitute the simplest and least reactive class of organic compounds because they only consist of hydrogen and carbon with sp³ hybridization, and they lack reactive functional groups. Although alkanes undergo reactions such as thermal cracking and combustion, they are much less reactive than other classes of compounds that contain functional groups.
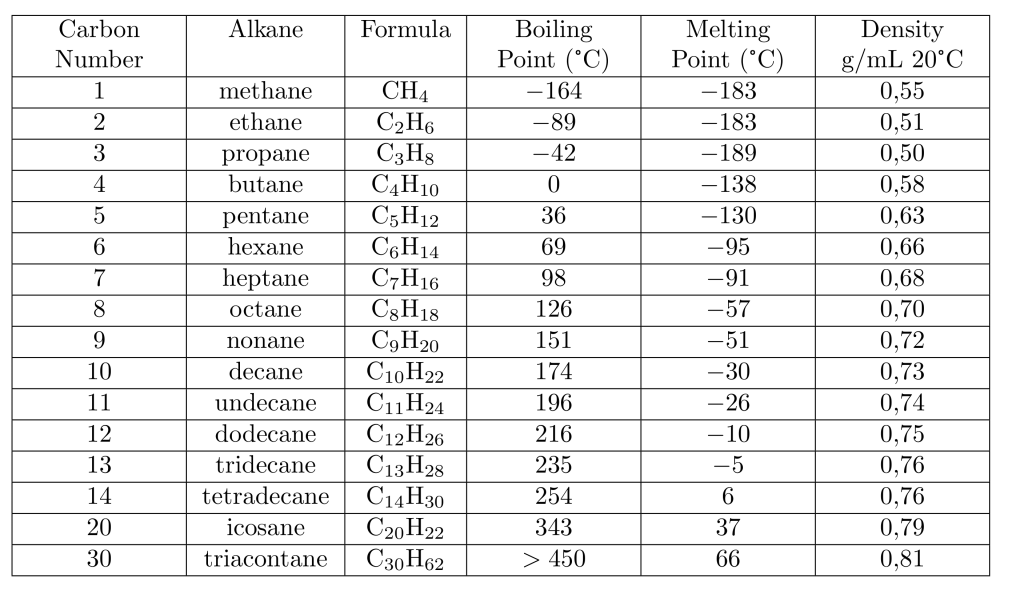
Physical properties of some unbranched alkanes, called n-alkanes
Alkanes are nonpolar; they are considered hydrophobic (“water-repelling”) because they do not dissolve in water. They dissolve in less polar organic solvents. The density of n-alkanes is close to 0.7\frac{g}{mL}, and since they are insoluble in water, a mixture of alkanes like gasoline with water separates quickly into two phases, with the alkane remaining on the upper layer.
The boiling points of alkanes gradually increase as the number of carbon atoms and molecular masses increase. Larger alkane molecules have a greater surface area, leading to stronger van der Waals intermolecular interactions. These interactions prevent vaporization and boiling.
Similar to boiling points, melting points also increase with molecular mass. However, alkanes with an even number of carbon atoms pack more efficiently in solid structures, requiring higher temperatures to melt. Alkanes with an odd number of carbon atoms do not pack as well and melt at lower temperatures.
Alkane Nomenclature
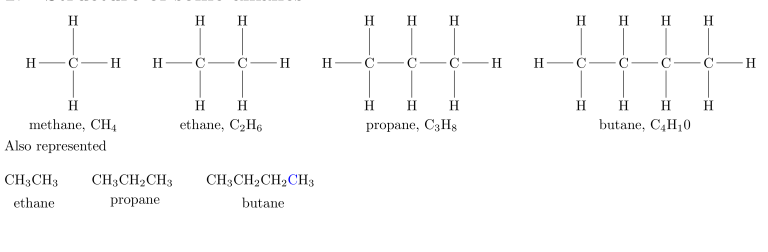
The names methane, ethane, propane, and butane have historical roots. Starting from pentane, alkanes are named using the Greek prefix corresponding to the number of carbon atoms, plus the suffix -ane to identify the molecule as an alkane.
Common names
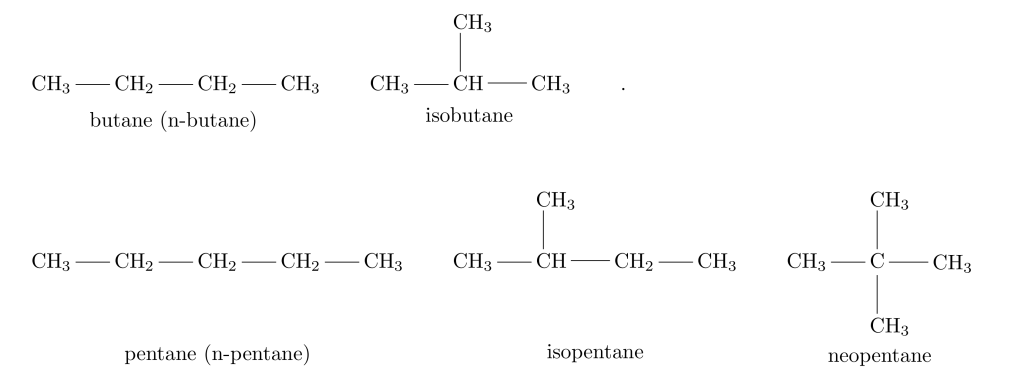
Most alkanes have positional structural isomers, and we need a way to name the different isomers. For example, the three isomers of C_{5}H_{12} are known as pentane (or n-pentane), isopentane, and neopentane. These are common names or trivial names, meaning they are historical names that arise from common usage. Trivial names cannot easily describe larger molecules with more isomers.
Systematic names or IUPAC names
In 1892, a group of chemists gathered to devise a system for naming compounds; this marked the first meeting of the group later known as the International Union of Pure and Applied Chemistry (IUPAC). The names generated using the rules devised by IUPAC are known as IUPAC names or systematic names.
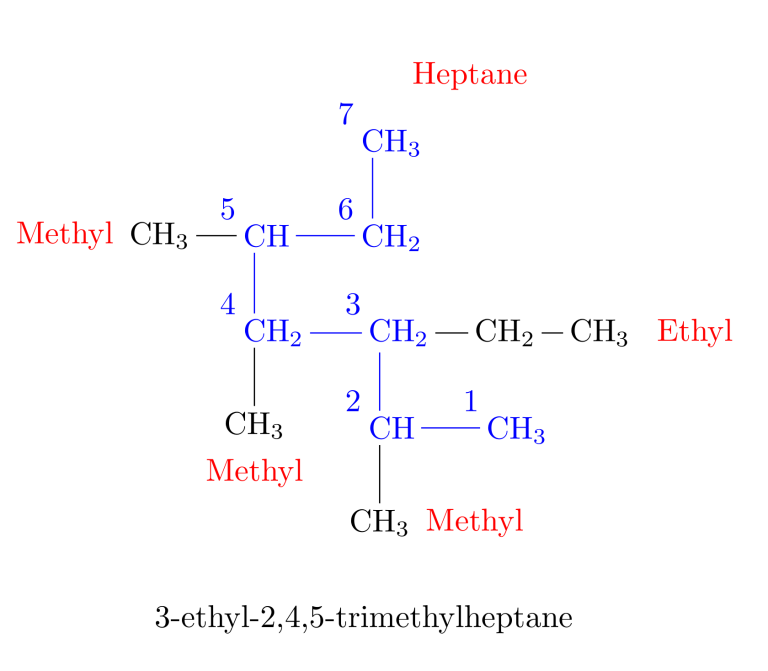
Rule 1: The Main Chain -> Find the longest continuous chain of carbon atoms and use the name of this chain as the base name of the compound.
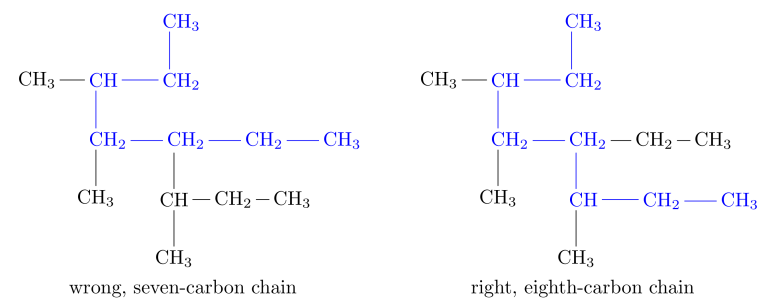
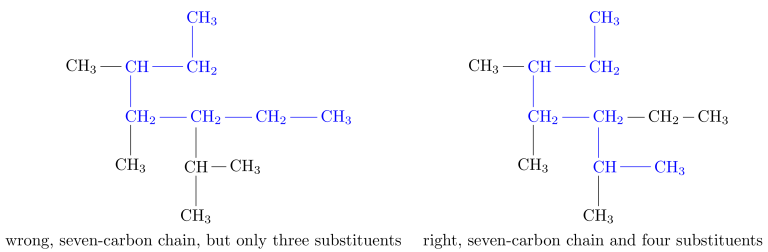
The groups attached to the main chain are known as substituents as they substitute for hydrogen atoms. When there are two longest chains of equal length, use the chain with the greater number of substituents as the main chain.
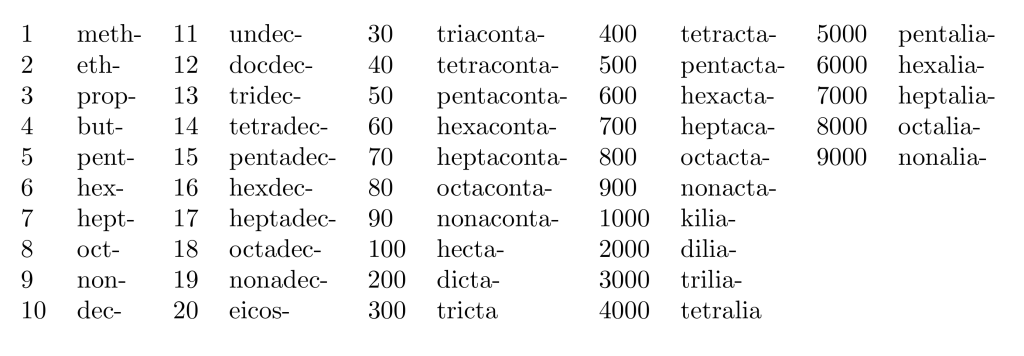
The numerical terms for use in hydrocarbon names or as multiplying prefixes are:
According to the list, the prefix for the main chain would be “heptane” (hept = 7 carbon atoms in the main chain).
Rule 2: Number the Main Chain -> Number the longest chain, starting from the end of the chain closest to a substituent.
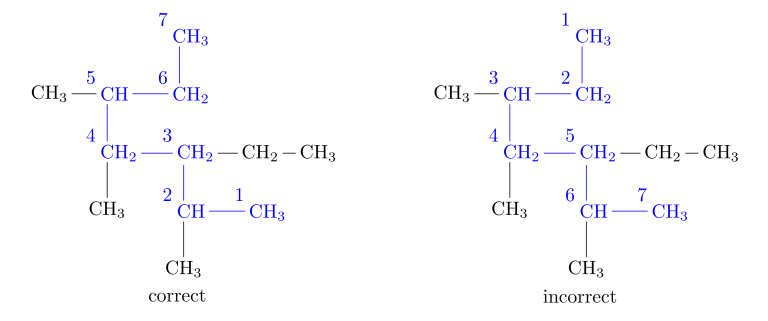
Rule 3: Name the Alkyl Groups -> Name the substituent groups attached to the longest chain as alkyl groups. Indicate the position of each alkyl group by the number of the carbon atom in the main chain to which it is attached.
Alkyl groups are named by replacing the -ane suffix of the alkane name with -yl. Methane becomes methyl, ethane becomes ethyl, and so on.
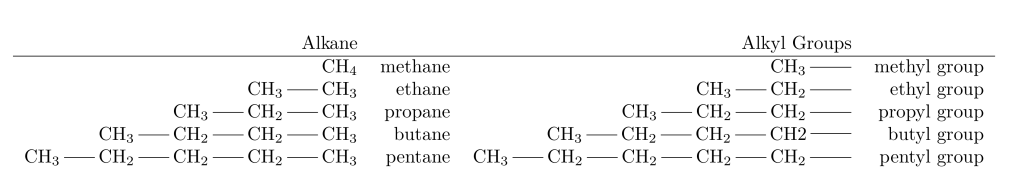
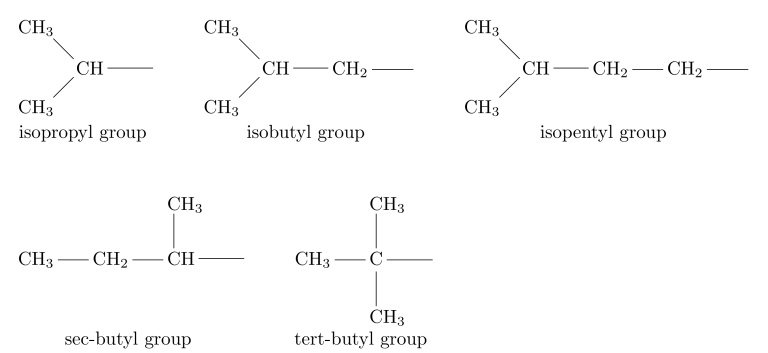
The terms “secondary-butyl” (sec-butyl) and “tertiary-butyl” (tert-butyl or t-butyl) are derived from the level of alkyl substitution of the carbon atom linked to the primary chain. In the sec-butyl group, this carbon atom is secondary (2°), meaning it is connected to two other carbon atoms. Conversely, in the tert-butyl group, it is tertiary (3°), indicating it is connected to three other carbon atoms.
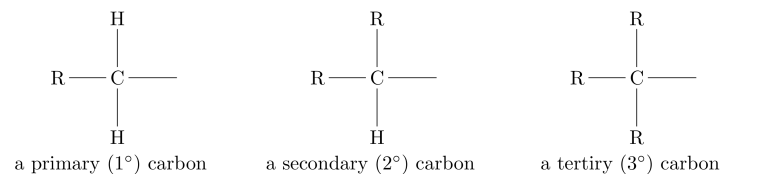
The names of the substituents are:
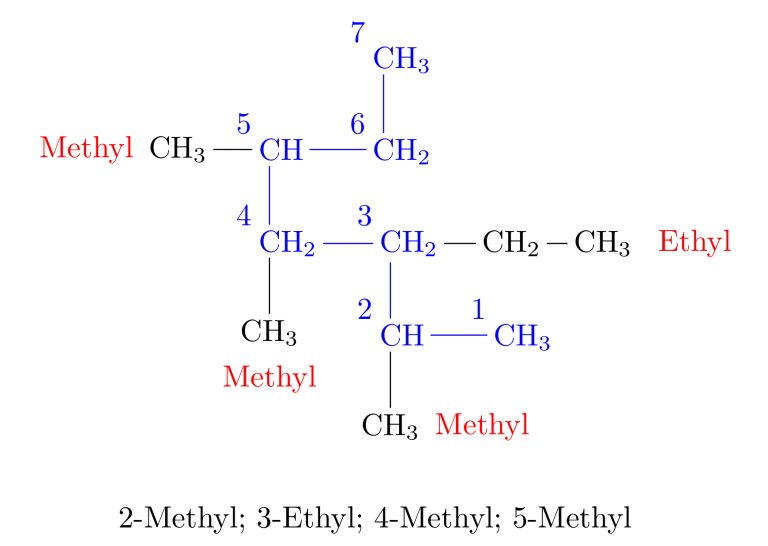
Rule 4: Arrange Multiple Groups -> When two or more substituents are present, list them in alphabetical order. If there are two or more identical alkyl substituents, use the prefixes di-, tri-, tetra-, etc., to avoid repeating the alkyl group’s name.